Copyright © 1994 by Annual Reviews. All rights reserved
| Annu. Rev. Astron. Astrophys. 1994. 32:
153-90 Copyright © 1994 by Annual Reviews. All rights reserved |
Must we, as Solon advises, always keep the goal insight?
Aristotle, Nichomachean Ethics
The s-process is the other major nucleosynthetic process that assembles heavy elements. We know that the s-process path in the neutron number-proton number plane crosses the neutron closed shells at the valley of beta stability. This tells us that the s-process occurred in an environment with a much lower neutron density than the r-process. Also, the s-process occurred over a much longer time period.
In this section we seek to understand how the s-process occurs. We then turn to the question of s-process sites. Finally we consider constraints on those sites.
Because of the neutron densities and timescales inferred for the s-process from the abundance peaks, we can infer that the s-process is not a freeze out from equilibrium. Instead, it is a neutron-capture process that occurs in a system striving to reach equilibrium, but falling short of its goal. The main reactions carrying the bulk of the nuclei towards the iron group can liberate neutrons. Pre-existing seed nuclei capture these neutrons and produce the s-nuclei. The s-process is clearly a secondary process.
The dominant reactions that can liberate neutrons are
13C( ,
n)16O and
22Ne(
,
n)16O and
22Ne( ,
n)25Mg. In these reactions, the
neutron-rich isotopes,
13C and 22Ne give up their excess neutrons to heavier
nuclei. At this
point, we may ask where these excess neutrons came from in the first
place. The answer to this interesting question illustrates an
important point about the overall nuclear evolution of the universe.
,
n)25Mg. In these reactions, the
neutron-rich isotopes,
13C and 22Ne give up their excess neutrons to heavier
nuclei. At this
point, we may ask where these excess neutrons came from in the first
place. The answer to this interesting question illustrates an
important point about the overall nuclear evolution of the universe.
The abundances that emerge from the Big Bang are roughly 90% by number 1H and 10% 4He (e.g. Walker et al 1991). This yields Ye = 0.88. On the other hand, we may make the observation that 1H and 4He are the only proton-rich (that is, with proton number greater than neutron number) stable isotopes in nature. This means that in order for nature to put the nucleons in the universe into nuclei with the strongest binding energy per nucleon (iron-group nuclei), the Ye of the universe must decrease.
Most of the decrease in Ye comes from the weak decays
in the p - p
chains and the CNO cycle during hydrogen burning. These interactions
drop Ye from 0.88 to 0.5 in material that has
completed hydrogen
burning. 4He itself does not have any excess neutrons, but some
production of excess neutrons occurs in the CNO cycle due to reactions
like 12C(p,
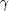 )
13N(
)
13N(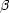 +)13C. The
net result is the conversion of a free
proton into an excess neutron, and a drop in Ye. The
22Ne production
builds up from abundant 14N produced in the CNO cycle. The
sequence is
14N(
+)13C. The
net result is the conversion of a free
proton into an excess neutron, and a drop in Ye. The
22Ne production
builds up from abundant 14N produced in the CNO cycle. The
sequence is
14N( ,
,
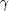 )
18F(
)
18F(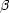 +)
18O(
+)
18O( ,
,
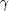 )
13Ne. Here it is the fact that the only
stable isotope of flourine is neutron rich that leads to a decrease in
Ye. We see that the excess neutrons in 13C
and 22Ne
are a consequence of the overall drive to decrease Ye in
stars. We must keep the goal of
the nuclei in sight to understand where the excess neutrons come from
that drive the s-process.
)
13Ne. Here it is the fact that the only
stable isotope of flourine is neutron rich that leads to a decrease in
Ye. We see that the excess neutrons in 13C
and 22Ne
are a consequence of the overall drive to decrease Ye in
stars. We must keep the goal of
the nuclei in sight to understand where the excess neutrons come from
that drive the s-process.
The first attempts to understand the details of the s-process led to
the classical model. The neutron density is always low in the
s-process (compared to the r-process). If a nucleus is unstable to
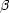 -
decay following neutron capture in the s-process, it will almost always
-
decay following neutron capture in the s-process, it will almost always
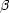 -
decay to the first available stable isobar before it can
capture another neutron. Thus, it generally suffices in s-process
studies to follow only the abundances as a function of mass number,
which only change by neutron capture. In this approximation, the rate
of change of the abundance NA of nuclei with mass
number A is
-
decay to the first available stable isobar before it can
capture another neutron. Thus, it generally suffices in s-process
studies to follow only the abundances as a function of mass number,
which only change by neutron capture. In this approximation, the rate
of change of the abundance NA of nuclei with mass
number A is

| (9) |
where nn is the neutron number density and
< v>A is the thermally
averaged neutron-capture cross section for the stable isobar of mass
number A. We can write
<
v>A is the thermally
averaged neutron-capture cross section for the stable isobar of mass
number A. We can write
< v>A as
v>A as
 A
vT, where vT is the thermal
velocity of neutrons and
A
vT, where vT is the thermal
velocity of neutrons and
 A is an
average cross section, given in
terms of vT. With the definition of the neutron exposure
A is an
average cross section, given in
terms of vT. With the definition of the neutron exposure

| (10) |
we find

| (11) |
Note that the neutron exposure
 is a fluence. It has units
of inverse millibarns (1 barn = 10-24
cm2). Because it is a neutron flux
integrated overtime, it is an appropriate evolutionary parameter for
the s-process. If the s-process achieves a steady state, then
dNA /
d
is a fluence. It has units
of inverse millibarns (1 barn = 10-24
cm2). Because it is a neutron flux
integrated overtime, it is an appropriate evolutionary parameter for
the s-process. If the s-process achieves a steady state, then
dNA /
d
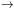 0 and
0 and
 A
NA
A
NA
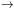 constant.
constant.
Clayton et al (1961) were able to show that a single neutron exposure r could not reproduce the solar system's abundance of s-only nuclei. Seeger et al (1965) showed that an exponential distribution of exposures, given by

| (12) |
where f is a constant and N56 is the initial abundance of 56Fe seed, did reproduce the solar distribution of s-nuclei. For the distribution of exposures given in Equation (12), Clayton & Ward (1974) found that for an exponential average of flows in the s-process

| (13) |
A fit to the empirical
 A
NA for s-only nuclei then gives the
quantities f and .
A
NA for s-only nuclei then gives the
quantities f and .
A complication to the above classical model is the branching that
occurs at certain isotopes. Here it may be that the
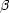 -
decay rate is
not considerably greater than the neutron-capture rate. In some cases
the nucleus may
-
decay rate is
not considerably greater than the neutron-capture rate. In some cases
the nucleus may
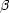 -
decay before neutron capture and in others it may
neutron capture before suffering
-
decay before neutron capture and in others it may
neutron capture before suffering
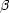 -
decay. The assumptions leading to Equation (9) thus break down.
Ward et al (1976)
developed an analytic
treatment of branching in the case of a time-independent neutron
flux. For time-dependent neutron fluxes, it is necessary in general to
solve a full network of nuclei numerically (e.g.
Howard et al 1986).
Since the s-process branchings will in general be temperature
and neutron density dependent, s-nuclei branchings are important
diagnostics of the environment in which the s-process occurred. We
will see this in more detail in Section 4.3.
-
decay. The assumptions leading to Equation (9) thus break down.
Ward et al (1976)
developed an analytic
treatment of branching in the case of a time-independent neutron
flux. For time-dependent neutron fluxes, it is necessary in general to
solve a full network of nuclei numerically (e.g.
Howard et al 1986).
Since the s-process branchings will in general be temperature
and neutron density dependent, s-nuclei branchings are important
diagnostics of the environment in which the s-process occurred. We
will see this in more detail in Section 4.3.