Copyright © 1994 by Annual Reviews. All rights reserved
| Annu. Rev. Astron. Astrophys. 1994. 32:
153-90 Copyright © 1994 by Annual Reviews. All rights reserved |
The Master said, "Ssu, I believe you look upon me as one whose aim is simply to learn and retain in mind as many things as possible." He replied, "That is what I thought. Is it not so?" The Master said, "No; I have one thread on which I string them all:"
The Analects of Confucius
The r-, s-, and p-processes are distinct nucleosynthetic mechanisms. They occur in different environments and under quite different conditions. Nevertheless, it is useful to seek some unifying concept by which we may understand these disparate processes. We will see that entropy is the concept we need. Careful consideration of entropy in the various nucleosynthetic processes will clarify our discussion and give us insight into how these processes occur and why they occur where they do. With this insight we will see how the r-, s-, and p-processes are each unique answers to the same question: "How does nature produce heavy elements?"
Let us begin our discussion by considering a given thermally isolated system at constant volume. The system has total energy E0. The entropy of this system is

| (1) |
where k is Boltzmann's constant and
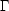 is the number of
energetically
allowed macroscopic states available to the system. By macroscopic
state we mean a particular distribution of the constituents of the
system among their single- particle quantum-mechanical states. In
essence, a macroscopic state of the system is one particular way the
constituents of the system can share the total energy
E0. Suppose the
system does not have all macroscopic states of energy
E0 available to
it. In this case, the entropy is less than its maximum. The system
will evolve by the Second Law of Thermodynamics and add more
macroscopic states to its repertoire. In this evolution, the entropy
will thus increase. The system will continue to evolve until all
macroscopic states of energy E0 are available to the
system. Once the
system reaches this point, it is at maximum entropy and experiences no
further evolution. The system has attained equilibrium. We thus see
that equilibrium, or maximum entropy, is the evolutionary endpoint of
any thermally isolated system.
is the number of
energetically
allowed macroscopic states available to the system. By macroscopic
state we mean a particular distribution of the constituents of the
system among their single- particle quantum-mechanical states. In
essence, a macroscopic state of the system is one particular way the
constituents of the system can share the total energy
E0. Suppose the
system does not have all macroscopic states of energy
E0 available to
it. In this case, the entropy is less than its maximum. The system
will evolve by the Second Law of Thermodynamics and add more
macroscopic states to its repertoire. In this evolution, the entropy
will thus increase. The system will continue to evolve until all
macroscopic states of energy E0 are available to the
system. Once the
system reaches this point, it is at maximum entropy and experiences no
further evolution. The system has attained equilibrium. We thus see
that equilibrium, or maximum entropy, is the evolutionary endpoint of
any thermally isolated system.
We can now use these considerations to ask what happens to the nucleons and nuclei in some nucleosynthetic environment. If the system of nucleons and nuclei is thermally isolated and out of equilibrium, it will evolve towards equilibrium. Given enough time, the system will reach equilibrium and attain maximum entropy. At this point, it is in nuclear statistical equilibrium (NSE), and it is a simple matter to compute the abundance of any nuclide (Burbidge et al 1957). We find (e.g. Meyer 1993) that, for a nuclear species of atomic number Z and mass number A, the abundance per baryon Y(Z, A) is
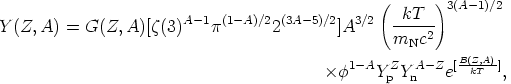
| (2) |
where G(Z, A) is the nuclear partition function,
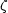 (3) is the
Riemann zeta function of argument 3, T is the temperature,
mN is the mass of a
single baryon,
(3) is the
Riemann zeta function of argument 3, T is the temperature,
mN is the mass of a
single baryon, 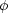 is the
photon-to-baryon ratio, Yp, is the abundance per
baryon of protons, Yn is the abundance per baryon of
neutrons,
and B(Z, A) is the binding energy of nucleus
(Z, A). We note that
is the
photon-to-baryon ratio, Yp, is the abundance per
baryon of protons, Yn is the abundance per baryon of
neutrons,
and B(Z, A) is the binding energy of nucleus
(Z, A). We note that
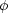 is given by
is given by

| (3) |
where 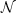 A is
Avagadro's number and p is the baryon mass
density. The binding energy of nucleus (Z, A) is
A is
Avagadro's number and p is the baryon mass
density. The binding energy of nucleus (Z, A) is
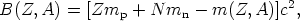
| (4) |
where N = A - Z and m(Z, A), mp, and mn are the masses of nucleus (Z, A), the proton, and the neutron, respectively.
From Equation (2), we see that the NSE abundance of nuclei is nonzero. This might at first be surprising. If we combine free neutrons and protons into nuclei, we decrease the number of free particles of the system. This would yield fewer ways of sharing the total energy of the system and thus decrease the number of macroscopic states available to the system. The nuclear reactions also release binding energy, however, which increases the number of photons in the system, the energy available to leptons, and the excitation energy in the nuclei. These effects increase the number of ways the system can share the total energy of the system and, hence, can increase the number of macroscopic states available to the system. This increase can more than compensate for the decrease in the number of states due to the loss of free particles and can lead to an increase in the entropy. Once the system has evolved to the point that it experiences no net increase in the number of macroscopic states by changing the abundance of any particular nucleus, the system has reached NSE.
Which nuclei dominate the abundance distribution in NSE? This
depends on the photon-to-baryon ratio or, equivalently, the entropy
per baryon since this latter quantity scales monotonically with the
photon-to-baryon ratio (e.g.
Meyer & Walsh 1993).
From Equation (2) we
see that the abundance of some heavy nucleus (Z, A) depends on
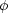 1-A. At
some given temperature then, the larger
1-A. At
some given temperature then, the larger
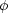 is, the smaller will be the
abundance of the heavy nucleus (Z, A). Fewer heavy nuclei
means more
light nuclei and free nucleons. The strong dependence of the NSE
abundances on
is, the smaller will be the
abundance of the heavy nucleus (Z, A). Fewer heavy nuclei
means more
light nuclei and free nucleons. The strong dependence of the NSE
abundances on 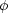 will be
crucial for the r-process. This dependence on
will be
crucial for the r-process. This dependence on
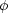 is apparent in
Figure 2. If we fix the temperature, the larger
is apparent in
Figure 2. If we fix the temperature, the larger
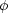 is, the more likely
nucleons are free or contained in light nuclei.
is, the more likely
nucleons are free or contained in light nuclei.