
This element was known in antiquity and used in brass. The name comes from the German name Zink.
Ionization energies
ZnI 9.4 eV, ZnII 18.0 eV, ZnIII 39.7 eV.
Absorption lines of ZnI
| Group | V | III | Ib |
| A2 | 0.03 | ||
| A7 | 0.015 | ||
| F0 | 0.075 | 0.036 | |
| F4 | 0.050 | ||
| F5 | 0.071 | 0.046 | |
| F6 | 0.069 | ||
| F8 | 0.060 | 0.114 | |
| G0 | 0.060 | ||
| G1 | 0.104 | ||
| G2 | 0.071,0.076 | ||
| S | 0.080 | ||
| G8 | 0.068(IV) | ||
| K0 | 0.085 | ||
| K2 | 0.082 | ||
| K5 | 0.067 | ||
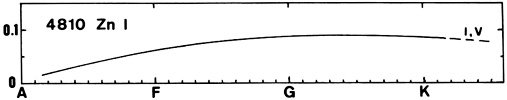
Zn I (see for instance the line at 4810) appears in A-type stars and grows slowly toward late types. No luminosity effect is visible.
 |
Absorption lines of ZnII
| Group | V |
| B5 | 0.095(IV) |
| B7 | 0.115 |
| A0 | 0.150 |
Source: Data are from Sadakane et al. (1988). |
|
Behavior in non-normal stars
ZnII has its resonance lines at 2025(1) and 2062(1). Sadakane et al. (1988)
studied the behavior of the 2062 line in Bp stars of the Hg-Mn
subgroup. The line can be very strong (W up to 0.560), weak or
absent.
They find no clear relation to the behavior of the neighboring elements
Cu and Ga. Cowley et al. (1974) detected Zn in Ap stars of the
Cr-Eu-Sr subgroup in the photographic region.
Zn is strong in Am stars, the W values being stronger by a factor of about two than in normal stars of the same temperature (Smith 1973, 1974).
Sneden etal. (1991) found that Zn behaves like Fe and Ni in all metal-weak stars (disk and halo types). It can thus be considered a typical metal. Zn was detected in one iron-deficient (Fe / H = -5 dex) population two A-type star. This element does not share the iron abundance (W(4810, ZnI) = 0.030) (van Winckel et al. 1992).
Isotopes
Zn has five stable isotopes, namely Zn 64, 66, 67, 68 and 70, which in the
solar system occur with frequencies of respectively 48%, 28%, 4%,
19% and 1%. There also exist ten short-lived isotopes and isomers.
Origin
All five Zn isotopes can be produced by the statistical equilibrium
process. Zn 67, 68 and 70 can also be produced by the s process and
Zn64 by explosive nucleosynthesis.
Published in "The Behavior of Chemical Elements in Stars", Carlos Jaschek and Mercedes Jaschek, 1995, Cambridge University Press.